Introduction :
dл- рл BONDS. There are several structural phenomena that have traditionally been attributed to the formation of dл- рл Воnds. Recent work has raised some doubts. The phenomena in questions are exemplified by :
1. The fact that for amines such as (R3Si)2 NCH3, (R3Si)3 N and (H3Ge), N, the central NS₁₂C, NSi, and NGe, skeletons are planar.
2. Many tetrahedral species such as SiO, PO3, SO2 and CIO have bond lengths shorter than those predicted from conventional tables of single bond radii. In silicates the Si-O-Si units also show what were considered to be Si-O distance that are "too short" for single bonds.
Recent re-examinations of these phenomena by both theoretical and experimental methods together with earlier arguments now suggest that the dл- рл contributions to these effects are at best small. Thus, in reading literature written prior to 1985, where such interactions are often accorded great importance, one should now be sceptical of all but the facts themselves.
This is not to say that dл - pл bonding in main group compounds is never important. Probably in the case of SNS units, and in F3F = N, where the SN distances are very short indeed. However, it is always dangerous to attribute all structural effects to simple orbital overlaps, even if the explanation seems to fit, and the rise and fall of the dл - рл overlap hypothesis is a case in point.
In a multiple bonded molecule having bond pairs + lone pairs = 4, 5 or 6, the bonds will be pл dл bond. In this case the central atom uses all its p-orbitals for hybridisations and has only d-orbitals available to overlap with p-orbitals of the adjacent atom to give dл- рл bоnd.
The formation of dл - рл bоnd is common for all the second period elements and is not important for the elements of third and higher periods. The рл-dл bonding is more favourable than the dл - рл bonding for higher atoms of third and higher periods.
dл- рл Bonding in Phosphorous Group Elements :
Phosphine Oxide, R3P = 0, presents an important example of the participation of d-atomic orbitals of nonmetallic elements in a bonding. Presence of л bonding is defected with the help of the evidences, such as reduction in the bond length, increase in the bond strength and the stabilisation of charge distribution. On these grounds, compared to
ammine oxide, phosphine oxide presents a strong evidence of the presence of dл- pл bond, in a very high stability of P = 0.
Similarly the fact that almost all t-phosphines are readily oxidised into RP = 0, also indicates that dл- рл bоnd is present in the P = O bonding. This is supported by the lower dipole moment of triethyl phosphine oxide (1.4 x 103 Cm. cf 16.7 x 103 Cm. of trimethyl amine oxide), higher dissociation energy of P = O bond (500-600 KJ, cf 200 300 KJ of N-O bond) and the smaller P-O bond lengths in phosphoryl compounds.
dл-рл Воnding in Nitrogen, Oxygen and Sulphur Compounds :
There are number of examples, which show dл- рл bonding in nitrogen, oxygen and sulphur compounds:
(i) Mobile bonding in trisilyl amine results in the resonance in the molecule :
(ii) The bond angles in disiloxane, HSiOSiH3, and silyl isothiocyanate, H3Si-N-C-S indicates рл dл back-bonding (in comparison to ether and methyl isothiocyanate) :
The argument given against the use of d atomic orbitals in bonding by non-metallic elements is that a very high excitation energy is required for the same. Hence it may be concluded that the use of d-atomic orbitals in bonding by non-metallic elements will be possible only in their higher oxidation states and when they are linked with strong electronegative elements, eg. PF5,SF6OPX3 etc.:
(iii) The NS bond length in N=SF, indicates. The bond order = 2.7 indicating dл - рл bonding :
(iv) In S4N4F4 also there is indication of di- pi bonding (compare with (ii) S4H4N4) :
(v) In diphenyl phosphonitrilic fluoride, there is evidence of bonding in the ring :


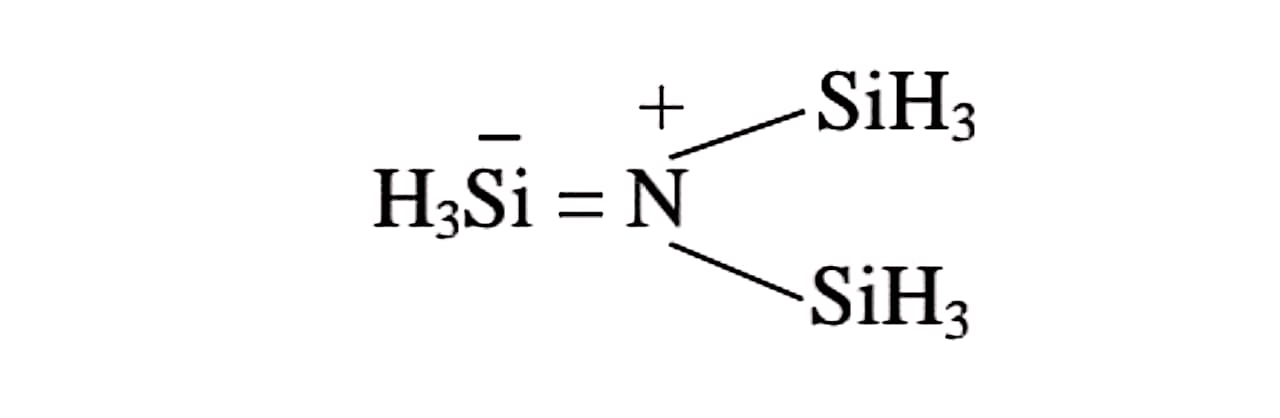





Post a Comment