In order to predict the geometry of covalent molecules, Valence Shell Electron Pair Repulsion Theory is used. This theory was given by Gillespie and Nyholm. According to this theory the geometry of a molecule depends upon the number of bonding and non-bonding electron pairs in the central atom. These arrange themselves in such a way that there is a minimum repulsion between them so that the molecule has minimum energy (i.e. maximum stability).
Gillespie Laws :
The following rules have been reported by Gillespie to explain the shape of some covalent molecules :
1. If the central atom of a molecule is surrounded only by bonding electron pairs and not by non-bonding electron pairs (lone pairs), the geometry of the molecule will be regular.
In other words we can say that the shape of covalent molecule will be linear for 2 bonding electron pairs, triangular for 3 bonding electron pairs. tetrahedral for 4 bonding electron pairs, trigonal bipyramidal for 5 bonding electron pairs:
| Name Of Compound | Bonding Electron Pair | Shape |
|---|---|---|
| BeCl2 | 2 | Linear |
| BeCl3 | 3 | Triangular Planar |
| SnCl4 | 4 | Regular Tetrahedral |
| PCI5 | 5 | Trigonal bipyramidal |
| SF6 | 6 | Regular Octahedral |
| Molecules | No. of Lone pairs on central atom | Bond Angle | Contraction in bond angle w.r.t. CH4 |
|---|---|---|---|
| CH4 | 0 | 109.5° | 0 |
| NH3 | 1 | 107.5° | 2° |
| H2O | 2 | 105.5° | 4° |
Applications of Gillespie Laws :
Let us take some examples in support of these laws:
(a) AX2 molecule, which has only two bond-pairs, will be linear:
X-A-X
Examples in this groups will be BeCl2, CaCl2, CO2 etc.
(b) If the molecule is AX3 (I) or AX, with a lone pair of electrons on the central atom A,
i.e. AX2E (II), then the molecule will be triangular
(c) If the molecule is AX, (III) or AX3E (IV) or AX2E2, then AX, will be tetrahedral; AXE will be pyramidal and AX2E2 will be angular. (Fig. 1.2):
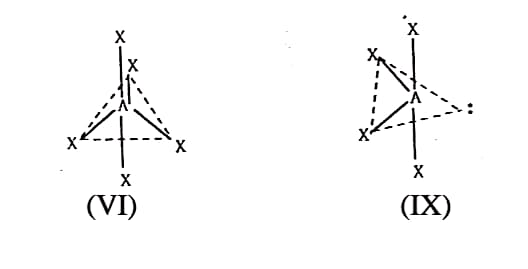
(d) If the molecule is AX, (VI) or AXE (VII) or AX3E2 (VIII) or AXE (IX) then AX, will be triangular bi pyramidal; AXE will irregular tetrahedral; AX3E2 will be T-shaped,; and AXE, will be linear. (Fig. 1.3)
(e) If the molecule is AX, (X) or AX-E (XI) or AXE2 (XII) then AX, will be octahedral, AX-E will be square pyramidal; and AXE2 will be square planar. (Fig. 1.4)Comparison of CH4, NH3, H₂O and H3O+
In table 1.1 bond angles in CH, NH3 and H₂O molecules are given. In all these molecules, the central atom (C. N and O respectively) is sp³ hybridised. But they differ in the number of lone pair (s) present on the central atom, being zero in CH4, one in NH3 and two in case of H₂O. Thus the repulsive force between electron pairs gradually increases in these molecules from CH4 to H2O, resulting in the change of geometry and the bond angles. This CH4 (Four bond pairs) is tetrahedral with the
SF4 molecule has 4 bond-pairs and one lone pair on the central S atom. The lp in this molecule has two options- it can sit in a axial or in an equatorial orbital. In the axial position (Fig. 1.6 (b))i)) it has three bps at 90° and one bp directly opposite to itself. While in equatorial position (Fig. 1.6 (b)(ii)) it has two bps at 90° and two bps at 120°. As in the equatorial position lp-bp repulsion is less and expansion is easy the lp prefers the equatorial position and the molecule is therefore irregular tetrahedral.
In CIF., the two lps may be axial-axial (Fig 1.6(c)(i)), or axial equatorial (Fig 1.6(c)(ii)), or equatorial-equatorial (Fig 1.6(c)(iii)) positions. As the axial position will result in maximum repulsion hence the axial position for the Ip is ruled out. Thus the molecule will have T. shaped geometry, according to the Fig. 1.6(c)(ii).
Similarly, due to reduced Ip-lp repulsions and a larger volume that a lp can occupy at equatorial positions, the [IC] ion will be linear. The three lps occupy the three equatorial positions, leaving the axial positions for the Cl atoms (Fig. 1.6(d)).
Limitations of VSEPR Theory :
1. This theory is not able to predict the shapes of certain transition element complexes.
2. This theory is unable to explain the shapes of certain molecules with an inert pair of electrons.
3. This theory is unable to explain the shapes of molecules having extensive delocalised π-electron system.
4. This theory can not explain the shapes of molecules which have highly polar bonds.





.jpg)

Post a Comment